Federal Government Announces Requirement for Reporting of Drug Shortages
Providing clear and concise information on drug shortages to help ensure the health and safety of Canadians
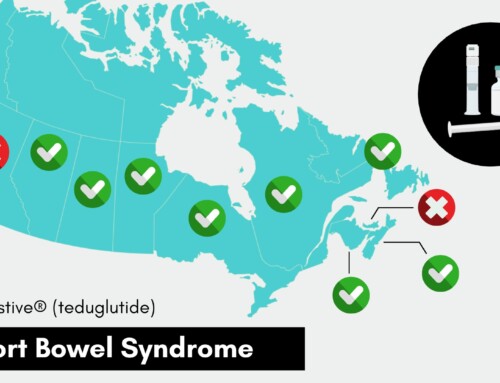
VANCOUVER, BC. TUESDAY, FEBRUARY 10, 2015 – At a media conference in Vancouver today, the Honourable Rona Ambrose, Federal Minister of Health announced that the Government of Canada is advancing regulations that will require manufacturers to report publicly any anticipated or actual drug shortages. By providing advanced warning of upcoming shortages, Canadians will be able to better proactively work with their health care professionals to find alternative options.
The pharmaceutical industry launched a voluntary reporting website, www.drugshortages.ca, in March 2012. While most manufacturers are cooperating with this initiative, some are not, leading to this need for regulation. In September 2013, Minister Ambrose announced a Protocol and Toolkit to help to prevent and mitigate the impacts of drug shortages.
To reflect and encourage industry accountability, Health Canada has launched a Public Notification Register listing all manufacturers that fail to voluntarily post their shortages. Once the reporting of drug shortages becomes mandatory, companies will face fines and penalties for failing to comply.
The new Public Register and the proposed approach to mandatory reporting will contain timely, comprehensive, and reliable information on actual and anticipated drug shortages. During the development of this new website and the regulations, manufacturers are still expected to voluntarily post information on all shortages on the industry-run website.
All stakeholders across the health care system have important roles to play in addressing drug shortages. Manufacturers, purchasers, provincial and territorial governments and health care institutions continue to have an important role in mitigating drug shortages and responding quickly to reduce the impact on patients. The Government of Canada is listening to Canadians and doing its part, and is calling on all stakeholders across the drug supply chain to do theirs.
The Gastrointestinal Society’s CEO, Gail Attara, who is also Chair of the Best Medicines Coalition (BMC), was a Witness before the Standing Committee on Health regarding this issue in 2013. “Today’s announcement on mandatory reporting of actual and anticipated drug shortages by the Federal Government is a significant step in providing vital health information. Patients all over Canada will benefit from improved accountability and transparency on the timely reporting of shortages,” she said, following the media conference. “The Gastrointestinal Society and the BMC strive to ensure that Canadians have safe and timely access to medications, and this recent development will help address some of the issues related to continuity of patient care and safety.”