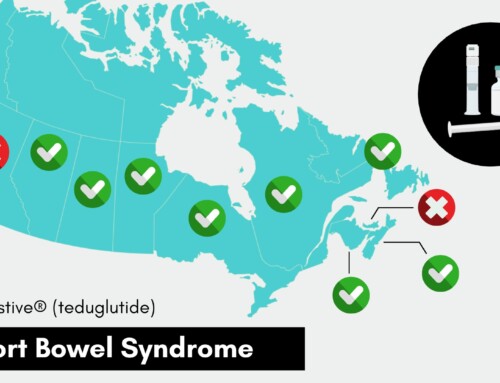
Access to Innovative Medicines for Canadian Patients
The Gastrointestinal Society continues to advocate for system-wide improvements in care and treatment for people with GI and liver diseases and disorders, ensuring that all patients have affordable and timely access to appropriate healthcare and medication. As part of this mandate, we have actively been involved in consultations with the Patented Medicine Prices Review Board (PMPRB) as they implement the regulatory changes made by the federal government in drug pricing. We have also collaborated with several patient coalitions to provide submissions to these ongoing discussions. See below for an explanation of the PMPRB and a brief look at the changes.
We appreciate that fiscal responsibility and proactive solutions are necessary. We know that decision-makers deal with rising costs of medications and for healthcare in general. However, we strongly caution PMPRB to consider the unintended consequences of the draft PMPRB Guidelines 2019 to innovation and timely access to medicines. This is a pressing concern when we think about the introduction of novel medicines essential for the wellbeing of patients, especially those who have chronic and/or rare diseases, as well as subsequent indications for existing medications. Drastic changes to the way prices are determined might delay access to life-extending and life-saving therapies or, in worst case scenarios, companies might not launch new products in Canada, thereby jeopardizing patient access to innovative therapies. The Gastrointestinal Society recognizes the trade-off between affordability and accessibility of new medicines and new regulations and guidelines that impede access to life-extending and/or saving medicines.
In 2018, the PMPRB established a multi-stakeholder Steering Committee on Modernization of Price Review Process Guidelines. Gail Attara, president and chief executive officer of the GI Society, was one of only three official patient group representatives to work with Health Canada and a variety of other healthcare stakeholders in providing in-person feedback over a series of months to the proposed guidelines. Gail also represented the Best Medicines Coalition during her time on the committee.
In 2018, we worked closely with the Best Medicines Coalition in developing a consensus document representing the perspectives of patient organizations from various therapeutic areas advocating for timely and equitable access to medicines for all Canadians. See our joint submission here.
In 2020, as members of the Better Pharmacare Coalition, the Gastrointestinal Society and the Canadian Society of Intestinal Research supported the organization in writing a submission to PMPRB, available here. The Better Pharmacare Coalition works together to call for appropriate access to evidence-based medicines that are proven effective and needed by patients in BC.
In addition to our efforts, the GI Society worked closely with the Alliance for Safe Biologic Medicines – an international cohort of patient and physician organizations representing appropriate and safe access to biologics and biosimilars, of which we are a member – to prepare a submission to the PMPRB about our concerns. See our joint submission here.
As discussions and changes evolve, we will continue to encourage governments and stakeholders to adopt appropriate regulations, best practices, and guidelines. It is imperative that decision-makers collaborate with patient groups, such as ours, to deliver programs directed at ensuring positive health outcomes for Canadian patients.
We will be providing more information about what the PMBRB changes mean for patients in the coming weeks.