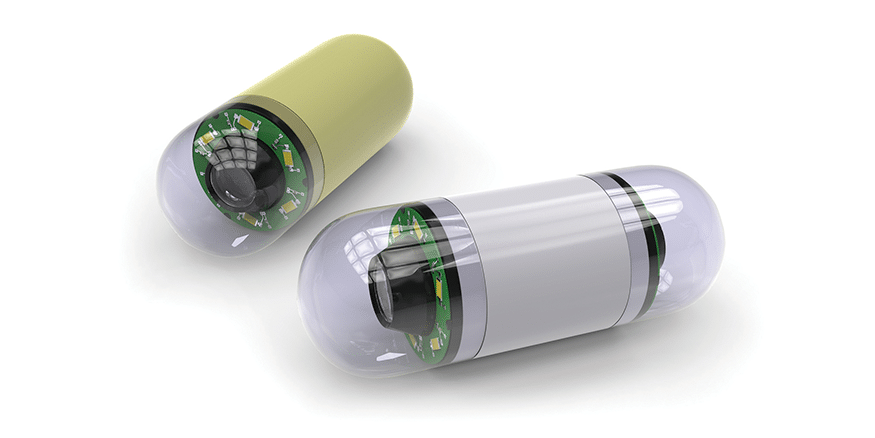
Capsule Endoscopy
Capsule endoscopy refers to the ability to assess the gastrointestinal tract with a wireless capsule. The test is primarily designed for assessing a variety of small intestinal disorders. Approved for use in Canada and the United States in the fall of 2001, capsule endoscopy has rapidly gained acceptance as an important diagnostic advance in small bowel evaluation.
Patients are prepared for the test with a laxative to ‘purge’ the intestine to ensure that the small intestine is clean so that images are adequate. A recorder is fastened to the patient’s waist and is worn for the entire test. A capsule is activated and swallowed. The capsule has within it a window, light, battery and transmitter. It transmits one image to the data recorder (worn about the waist) every 0.5 seconds. The battery-life of the capsule is approximately 8 hours, therefore allowing adequate time for the capsule to traverse the entire esophagus, stomach, and small intestine. During this entire time, the capsule transmits images to the data recorder. At the end of the 8 hours, the data recorder is returned and downloaded to a computer workstation.
The capsule is disposable and passes in the stool. The images are reviewed at the computer workstation in video format with tools available to speed up and slow down the review process, much like watching a VHS video. The review process is performed by the physician and usually takes about 1 hour. The patient typically swallows the capsule in the morning, is free to ambulate through activities of daily living through the day, and returns 8 hours later when the recorder is removed.
The test is primarily designed for small intestinal visualization in patients with recurrent bleeding for which a source can’t be found on standard investigations. Standard investigations would include an upper endoscopy, colonoscopy and small bowel x-rays (barium follow through). In patients with suspected small bowel pathology who have negative standard tests, capsule endoscopy can be considered. It must be emphasized that most patients do not require capsule endoscopy since their source of disease can be diagnosed with standard modalities available to us. The most common indication for capsule endoscopy is recurrent bleeding. In carefully selected patients the yield of this test is approximately 50%.
The second most common indication for this test is assessment of the small bowel for inflammatory bowel disease. Occasionally, patients with Crohn’s disease have negative studies and no evidence of disease on routine investigations, so capsule endoscopy is used to assess the small bowel. Only after a standard and thorough endoscopic investigation, is capsule endoscopy considered in this setting. It can be very helpful; however, strictures within the small intestine must be excluded prior to capsule studies. Occasionally, a capsule may become ‘stuck’ within a narrowing of the bowel and this may require surgical removal. This is a rare occurrence and has occurred in 4 patients of the first 120 capsules that we performed. In several of these patients, lesions that caused the capsule to obstruct were unrecognized by the referring physician and visible endoscopically. This emphasized the importance of thorough endoscopic assessment prior to capsule studies to avoid this complication.
Other ‘downsides’ to capsule endoscopy include the cost. It is an expensive test with disposable capsules. The expense is primarily with the capsule itself. On the other hand, repeat endoscopy for patients with recurrent bleeding is also expensive and in select patients, may not be beneficial. This new technology is very helpful for small bowel lesions and demonstrates findings that other tests may not be able to image. It is designed only for the small bowel and will not replace endoscopy of the upper GI tract (esophagus, stomach and duodenum) or the colon. It is an exciting advance that is extremely helpful for select patients.

