Food & Microbes: A Lifetime Commitment
An analysis of what the proposed Canada’s Food Guide changes mean for our bacterial inhabitants.
We are as much bacteria as we are human; current estimates suggest that we harbour an equivalent number of bacterial cells as we do human cells.1 Our resident bacterial cells – in addition to other microbes such as viruses, fungi, and yeast – comprise our microbiome. We depend on our intestinal microbiome for functions integral to our health, such as the break down of food otherwise indigestible by humans for us to use as energy, the production of vitamins, contributing to our immune systems, and protecting us against infection by harmful bacteria, such as those that cause food poisoning (pathogens).2
Our understanding of the composition and function of the human microbiome has advanced dramatically in recent years through the use of complex DNA sequencing technologies.3 Much of the analysis in defining a healthy microbiome has focused on bacterial populations, as bacteria form the largest component of our microbiome, far outnumbering the other microbes. The intestinal microbiome has been the site of most vigorous investigation, with alterations in bacterial composition and diversity (dysbiosis) at this site associated with numerous diseases including, but not limited to, inflammatory bowel disease (IBD), irritable bowel syndrome, asthma, allergies, obesity, and diabetes (type 1 and 2).4 Associations between diseased states and intestinal bacterial dysbiosis have further fueled the drive to understand factors influencing bacterial populations and, in turn, the effect of these changes on our intestinal health.
The Intestinal Ecosystem
The gastrointestinal tract, our largest interface with the external world, forms a tube through the body from mouth to anus. Our intestines comprise the largest portion of the gastrointestinal tract and serve the remarkable tasks of digesting the food we eat, absorbing digested nutrients, and forming a competent physical and immune barrier, all while housing trillions of resident bacteria.5 The intestinal microbiome resides within the inner portion of the tube through which food and digested materials pass (lumen). The bacterial populations able to inhabit different physical spaces along the length of the intestines are largely driven by environmental factors, including products released by intestinal cells, and the food we eat.6
The number of bacteria residing in the gastrointestinal tract increases moving from the upper small intestine (duodenum→jejunum→ileum) toward the large intestine or colon.6 The duodenum, where stomach contents pass first, contains bacteria numbering in the hundreds. In contrast, the regions of the colon farthest from the small intestine harbour trillions of bacteria. This increase in density is driven by several environmental factors including pH, which increases (becomes less acidic) toward the colon. Oxygen also affects bacterial density, as its presence is, in fact, toxic to many of our resident bacteria, and its concentration decreases toward the colon. Further, transit time of digested materials through the intestine, which is much faster through the small intestine than it is through the colon, affects the ability of bacteria to successfully maintain their presence.
The epithelial cells that interface the lumen and underlying intestinal tissue can also produce factors that affect bacteria.5 For instance, in the small intestine a type of intestinal epithelial cell (Paneth) is present that is absent in the healthy colon.7 Paneth cells are potent producers of antimicrobial peptides, which they release into the lumen, limiting bacterial populations. They are most numerous in the duodenum, decreasing in frequency in the ileum.
In contrast, another intestinal epithelial cell type responsible for producing intestinal mucus, the goblet cell, increases in frequency toward the colon.7 As a result, the thickness of the mucus layer increases toward and is at its thickest in the colon, while more diffuse in the upper small intestine. A diffuse layer of mucus in the duodenum and jejunum is necessary to ensure optimal absorption of nutrients as compared to the thicker mucus layer of the colon, which protects the intestinal epithelium from the compact feces moving through the lumen while also providing a place for resident bacteria to settle down or adhere in greater number.8
Intestinal Bacteria Depend on us for Food
The second major factor affecting bacterial populations within the intestine is the food that we eat. Just as we require energy created from the nutrients we absorb for our cells and (by extension) our bodies to function, bacteria require nutrients to survive. In the human intestine, specific nutrients are absorbed at different points along its length. In turn, this process influences which nutrients are available to bacteria residing in the lumen. For instance, the majority of broken down products of digestible carbohydrates (simple sugars) are absorbed in the small intestine.6 Therefore, bacteria residing in the colon must be able to use complex carbohydrates that are indigestible by humans for energy, as the majority of simple sugars have been absorbed by this point. See Table 1 for a summary of where along the intestine we absorb different nutrients.
| Nutrients | Site of Absorption |
| calcium, iron, broken down products of carbohydrates (glucose), fat-soluble vitamins (A and D) | duodenum |
| fat, broken down products of carbohydrates (sucrose, glucose, lactose), magnesium, fat-soluble vitamins (A and D), proteins (amino acids) | jejunum |
| proteins (amino acids), magnesium, vitamin B12, iron | ileum |
| water, potassium, sodium chloride, byproducts of bacterial digestion | colon |
| Table 1.9 | |
The ability of bacteria to utilize available nutrient sources is dependent on the tools that they have to break them down to a consumable form. Darwin best illustrated this concept in the discovery and description of his finches on the Galapagos Islands. Darwin observed a group of roughly a dozen closely related species of birds with remarkable differences in the size and shape of their beaks, which allowed them to be highly adapted to their specific food source from island to island.10 For instance, those feasting on the seeds and pulp of fruit from cacti employed their long-pointed beaks to consume their food, whereas those with shorter beaks would eat seeds and insect larvae near the base of cacti. Rather than beaks, bacteria employ enzymes, or proteins that help them break down different nutrients to a useable form for energy.11
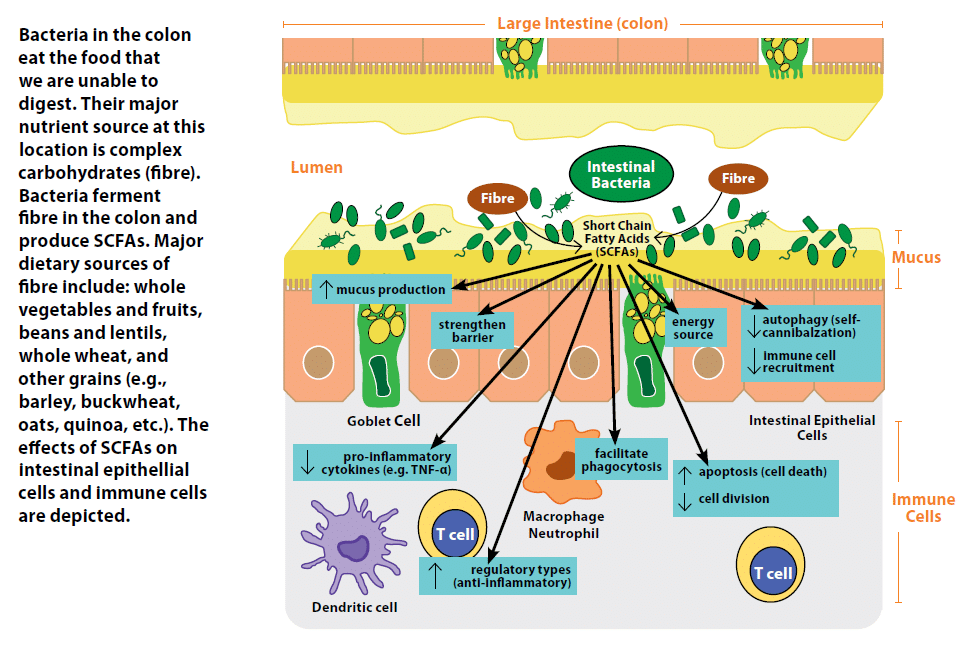
Through this process of breaking down and utilizing nutrients for energy, bacteria also produce many byproducts.2 These byproducts include gases, such as hydrogen and methane, vitamins such as K and the B-group (a source that we rely on), and metabolites such as short chain fatty acids (SCFAs), which our intestinal epithelial cells in the colon rely on as a major source of energy and can also serve to tone down the immune system.12
Short Chain Fatty Acids (SCFAs)
SCFAs are the end products of the metabolism of sugars in the absence of oxygen (fermentation). Bacteria in the colon are able to break down complex carbohydrates using enzymes such as polysaccharide lyases to a fermentable form, resulting in the production of large amounts of SCFAs.13 Bacteria who are able to feed off the abundant intestinal mucus in the colon using specialized enzymes such as glycosidases also produce SCFAs. The most abundant SCFAs are butyrate, propionate, and acetate, which serve as a major nutrient source for intestinal epithelial cells. Studies in rats born and housed in a sterile environment with no microorganisms living in or on them (germ free) indicate a 30% increase in food intake is required to get the same energy from ingested food in the absence of bacteria.14
In recent years, SCFAs have also been highlighted for their ability to shape immune responses and strengthen the intestinal barrier.15 In intestinal epithelial cells, SCFAs can induce improved intestinal barrier function by strengthening junctions between cells,16 enhance mucus production,17 decrease self-cannibalization of cells due to nutrient deprivation (autophagy) therefore maintaining the intestinal barrier,18 and decrease their release of signalling proteins (cytokines and chemokines) that recruit pro-inflammatory immune cells.2 SCFAs can also influence the immune cells underlying the intestinal epithelial cells. Exposure of one type of immune cell, termed a neutrophil, to acetate or propionate facilitates its ability to engulf and destroy (phagocytose) bacteria. In other types of immune cells, SCFAs suppress their ability to produce pro-inflammatory cytokines such as TNF-a, in the absence of any inflammatory stimulus.2 Furthermore, SCFAs can drive T cells, a type of white blood cell, to develop into a regulatory and anti-inflammatory type, while driving more reactive and inflammatory T cells to decrease their cell division and increase cell death (apoptosis).2
What all this means is that bacteria are very helpful to the digestive system.
Bacteria of the Human Intestinal Microbiome
At a high level, we can group bacteria into approximately thirty– although hypothesized to be closer to one hundred – formally described phyla, a term used to group together related organisms.19 In comparison, there are approximately thirty-six phyla that exist within the animal kingdom.20 The majority of bacteria composing the intestinal microbiome are represented within four of these bacterial phyla. In a healthy adult intestine, the majority of bacteria present (approximately 90%) are members of the Firmicutes and Bacteroidetes, with small numbers of Actinobacteria and Proteobacteria.21 In diseased states such as IBD, these ratios can change, resulting in decreased members of Firmicutes and increased Proteobacteria.
Focusing in on more closely related organisms within each phylum at the genus level, several bacterial groups have been recognized for their probiotic, or beneficial, effects. These include species of bacteria in the genus Lactobacilli, Bifidobacteria, and Roseburia, along with specific species such as Faecalibacterium prausnitzii, and Akkermansia mucinophila.22 Not surprisingly, many of the probiotic bacteria listed above are copious producers of SCFAs, which is one of the ways they exert their beneficial effects. Those with IBD have a decreased number of these beneficial bacteria and overall less bacterial diversity, with increases in organisms such as Escherichia coli, members of the phylum Proteobacteria.21
What do the Proposed Canada Food Guide Changes Mean for our Bacteria?
Fibre is one example of a complex carbohydrate that humans consume. Increased fibre intake has been associated with decreased cholesterol, as well as decreased risk of developing colorectal cancer.23 For our intestinal bacteria, which have the enzymes to digest fibre, increases in its intake translate to more food for them to ferment, resulting in greater production of SCFAs as byproducts. In humans, diets rich in dietary fibre have been associated with increases in beneficial bacteria such as Bifidobacteria and Lactobacilli. Consumption of concentrated supplements containing indigestible food ingredients that promote growth of beneficial bacteria (prebiotics) such as inulin might also increase populations of Bifidobacteria and F. prausnitzii. Prebiotics such as inulin are in foods such as green bananas, legumes, leeks, asparagus, and seaweed. On the other hand, lack of dietary fibre, in animal models, can lead to increases in intestinal bacteria that degrade and feed off of intestinal mucus, leading to a thinner mucus layer and potentially disrupted barrier.24
Protein recommendations in the new food guide encourage consumption of more plant-based proteins rather than animal-based protein sources. Reasons for this include an association between intake of red and processed meats and increased risk for colorectal cancer, in addition to soy protein being linked to lower cholesterol levels.23 One short-term study on human subjects published in the journal Nature examined the effects of exclusively animal- or plant-based diets on intestinal bacteria and found noteworthy results after only five days of consumption.25 Animal-based diets, composed of meats, eggs, and cheeses, resulted in greater numbers of inflammation-associated bacteria, as well as decreased members of Firmicutes, such as the genus Roseburia, that are capable of digesting plant fibres. In contrast, plant-based diets, rich in legumes, grains, vegetables, and fruits, had increased presence of Roseburia and F. prausnitzii, as well as nearly double the amount of SCFAs butyrate and acetate in their stool samples. Interestingly, the intestinal bacteria of the individuals consuming the animal-based diet returned to their original pre-diet bacterial structure in two days after discontinuation of the diet.
Fat comes in many forms, and there is evidence to suggest that its heavy consumption in the saturated form (those that are typically solid at room temperature) can lead to increased risk of cardiovascular disease.23 The effects of high-fat diets on intestinal bacteria have mostly been examined in animal models. In lab mice fed high-fat diets with a higher ratio of saturated fats, their proportions of intestinal bacteria change, with decreased Bacteroidetes and increased Firmicutes, as well as decreased overall diversity of bacterial species.26 In a rat model, a high-fat diet also led to reduced levels of SCFAs in stool samples.27
Sugar is well known for its association with increased risk for the development of diabetes and obesity.23 Sugar sweetened beverages consumed by children are also linked to increased fat deposition (adiposity).28 As a result, reduced sugar intake is recommended. Consumption of sugar and some artificial sweeteners in lab mice as well as in human subjects can lead to dysbiosis of the intestinal bacteria.29 Rats fed high-sugar diets showed increases in their intestinal E. coli populations.27 Interestingly, bacterial species associated with a high-fat and high-sugar diet in mice promote obesity in previously germ-free non-obese mice.30
Real-World Evidence
The best real-world evidence of the impact of diet on shaping the human intestinal microbiome comes from comparing populations with significantly different diets. In one such study, researchers compared intestinal microbiomes of children in a rural village of Burkina Faso to those of children living in Florence, Italy.31 The diet of the African children is low in fat and animal protein and composed largely of legumes, grains and vegetables, whereas the Italian children consume a diet higher in animal protein, sugar, starch, and fat, and much lower in fibre. The two cohorts of children displayed significantly different ratios of the major bacterial phyla making up their intestinal bacteria compared to one another, with the Italian children displaying similar changes to those observed in mice fed high-fat and high-sugar diets in the lab. Remarkably, the children in the African cohort harboured specific bacterial species, completely absent in the Italian children, that have enzymes capable of degrading a component of plant cell walls (cellulose) allowing maximal energy intake from plant fibres. They also displayed much greater bacterial diversity. In addition, major SCFAs such as butyrate and propionate were enhanced four-fold in the stool samples of the African children as compared to samples from Italian children. This study clearly highlights the impact of diet on the intestinal microbiome in a human population.
Conclusion
Overall, the changes to the new Canada’s Food Guide should have beneficial effects on our intestinal bacteria, with resulting benefits to our gastrointestinal health. The bacteria that stand to benefit most in the long term from increased consumption of vegetables, fruits, whole grain foods, and plant-based proteins are those that facilitate integrity of the intestinal barrier and the presence of anti-inflammatory immune cells. We are what we eat, and it seems, so are our bacteria.
